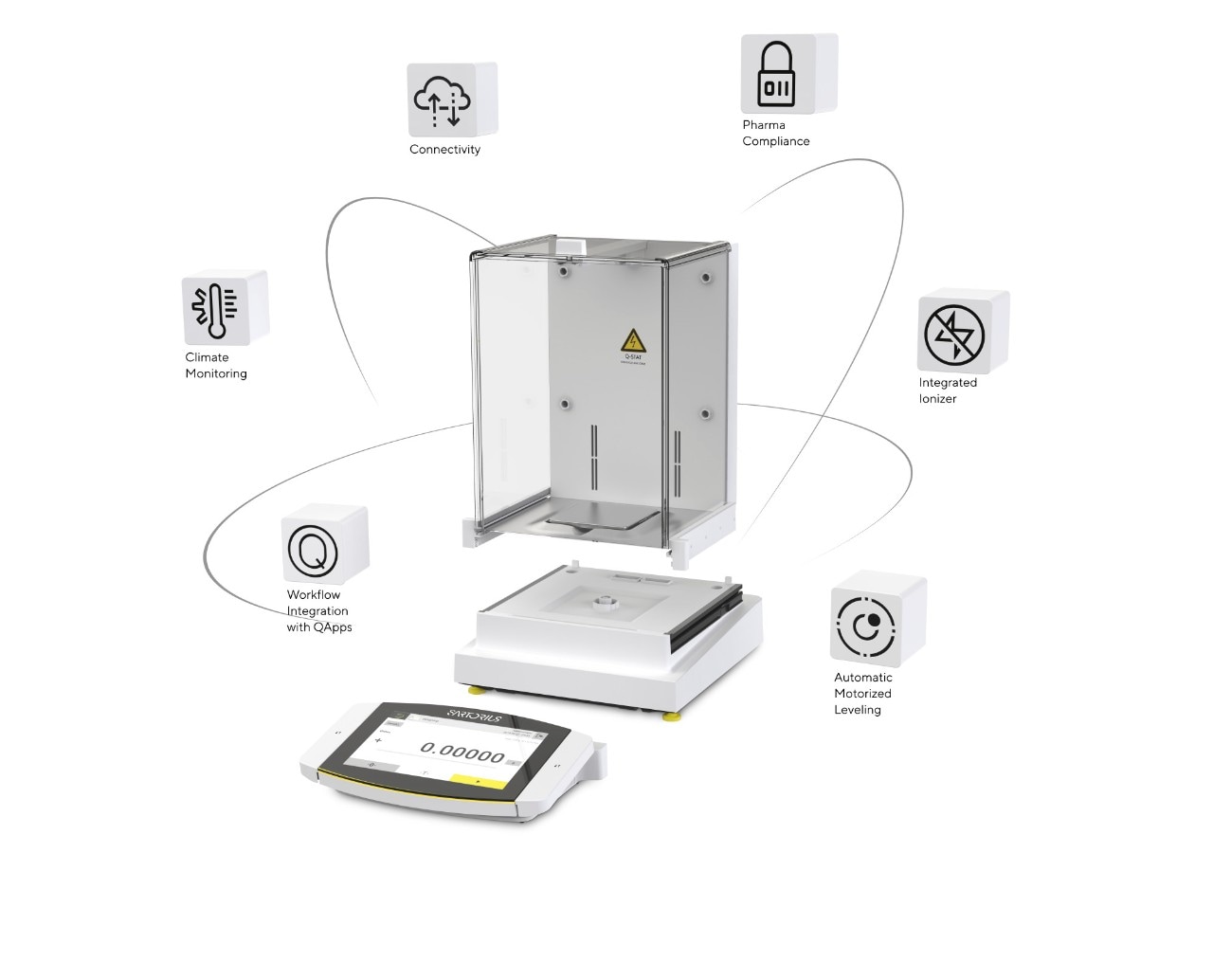
Maintain Data Integrity with Your Balance
If you want to avoid costly and reputation-damaging failed inspections, shut-downs or product recalls, maintaining data integrity is crucial. So why not choose a laboratory balance with the ALCOA principles built in?
By Mitesh Chhana and Sebastian Weber from Sartorius.
What is Data Integrity?
Data integrity refers to the maintenance and assurance of accurate data over a project's lifecycle. In the past, this meant transcribing data in handwritten notebooks or on ticket printouts combined with oversight and sign-offs to ensure credibility and robustness. Today, regulators encourage the use of modern instruments and software programs to ensure electronic data is recorded accurately and that recording remains accurate over time. To this end a wide range of industries, from biopharma to consumer care, have invested in digital solutions such as electronic lab notebooks (ELN's) or laboratory information management systems (LIMS).
"Failing to secure the integrity of your data leads to incorrect decisions, which, in industries like biopharma, can affect the quality of the product and, ultimately, the patient’s safety. In all regulated areas, the downstream errors caused by poor data integrity can lead to failed inspections, shut-downs, product recalls, and the need to repeat work – all of which are not only costly but also damage reputations."
Achieving data integrity means following the ALCOA (attributable, legible, contemporaneous, original, and accurate) principles, set out by the regulators. If your data is “attributable,” it means you can answer the who, what, where, and why questions associated with the data – who did what and when? Legible data has processes to ensure it has been permanently recorded in a readable format (in the past, that meant using special paper where the print wouldn’t disappear for at least 20 years, but electronics have made this easier). Contemporaneous data is documented at the time the work is performed with a timestamp – this is important because auditors want to drill down into the original data record, so you need processes in place that allow this. Finally, original and accurate data leaves an electronic signature tracking any changes made.
Automation is Key
Overall, the fewer manual steps in your data journey, the better – automation is the key, and this is true for all the data-generating instruments in your lab. One good example is the ubiquitous laboratory balance. The weighing performance of the laboratory balance is obviously important, but users should also consider the impact on compliance; does it make adhering to the ALCOA principles straightforward or difficult?
With the Cubis™ II balance, we put a great deal of thought into the software to achieve connectivity and compliance. For instance, the Cubis™ II has audit trails built in with alibi memory; it has access control and user management, allowing users to differentiate individual logins with different access levels for lab managers and trainees; it has e-signatures that eliminate the need for colleagues to manually verify results (removing the consequent workflow inefficiencies); and it integrates with LIMS, ELN's, or other companies’ IT systems. This latter point is important because it allows customers to deal with change easily – there’s no need to learn a new system or involve middleware because of the way it interfaces with preexisting LIMS workflows.
As you can see, the concept behind the Cubis™ II was to build data integrity into the instrument so that you can spend less time on validation and compliance. With that in mind, the Cubis™ II is compliant with the new Chapter 2.1.7 of the European Pharmacopeia and USP Chapter 41. The former became mandatory in January of 2022 and highlights the importance of calibration and documentation for measurement uncertainty. It is very similar to the USP Chapter 41 standard, but there are a couple of key differences; namely, symmetric errors may not exceed 0.05 percent in the European Pharmacopeia, while the limit is 0.1 percent in the USP; and though the minimum weight limits for both standards are identical, the measurement requirements are slightly different – the European standard requires a specific weight for repeatability tests. The Cubis™ II’s guided workflow takes the user through a checking step to determine the minimum sample weight and won’t weigh anything below the minimum required by the regulatory bodies.
Data integrity is a significant trend across industries, but the advice from auditors is somewhat limited. The Cubis™ II allows customers to cost-effectively implement ALCOA principles with minimal disruption to existing workflows, while also complying with the latest regulatory standards.
Mitesh Chhana is Regional Business Manager EMEA, Laboratory Weighing, and Sebastian Weber is Global Product Manager, Software Solutions, both at Sartorius.